- Review
- Open access
- Published:
T cell senescence and CAR-T cell exhaustion in hematological malignancies
Journal of Hematology & Oncology volume 11, Article number: 91 (2018)
Abstract
T cell senescence has been recognized to play an immunosuppressive role in the aging population and cancer patients. Strategies dedicated to preventing or reversing replicative and premature T cell senescence are required to increase the lifespan of human beings and to reduce the morbidity from cancer. In addition, overcoming the T cell terminal differentiation or senescence from lymphoma and leukemia patients is a promising approach to enhance the effectiveness of adoptive cellular immunotherapy (ACT). Chimeric antigen receptor T (CAR-T) cell and T cell receptor-engineered T (TCR-T) cell therapy highly rely on functionally active T cells. However, the mechanisms which drive T cell senescence remain unclear and controversial. In this review, we describe recent progress for restoration of T cell homeostasis from age-related senescence as well as recovery of T cell activation in hematological malignancies.
Background
The immune system plays a crucial role in the protection and fight against hematological malignancies and cancer [1,2,3]. Impairment of the immune system due to a decrease in immunological diversity of naïve T cells and an increasing number of senescent T cells with age leads to a higher susceptibility to disease and potentially promotes progression of malignant tumor in elderly [4, 5]. Moreover, human cytomegalovirus (HCMV) persistence occurs upon repeated T cell activation due to chronic infections with CMV and is considered a driver of immune senescence in humans, starting from puberty after thymic involution [6]. Nevertheless, cellular senescence can also act as a protective mechanism of the immune system against cancer by deactivating T cells which show excessive or aberrant proliferation [7,8,9]. T cell senescence is triggered in a variety of biological processes including tumor prevention, immune response to infections, and aging. It leads to distinctive phenotypic and functional alteration and can be caused by tumor-associated stresses, telomere damage, and regulatory T (Treg) cells [4, 10]. Here, we summarize recent findings of the role of senescent T cells in hematological malignancies as well as possibilities to restore function of senescent and exhausted T cells for immunotherapies, such as CAR-T cell therapy.
Discovery and concept of T cell senescence
Cellular immune senescence was firstly described in the late 70s and was mainly focused on age-dependent changes in macrophages and lymphocytes in mice. Previous findings show less influence of aging on macrophages, while lymphocytes show considerable changes during aging. Especially, T cells due to their relatively long lifespan of 4–6 months have time to mature and express different functions with age [11, 12]. Recently, immunosenescence and T cell senescence are described as the degeneration of innate and adaptive immunity and specifically as a depletion of naïve and effector T cells during aging. Nearing the end of their lifespan, T cells can become senescent, characteristically leading to a cell-cycle arrest while staying viable and metabolically active [13]. T cell senescence can be distinguished from T cell anergy and T cell exhaustion which share similar characteristics but have different origins. T cell anergy is a hyporesponsive state in T cells which is triggered by excessive activation of the T cell receptor (TCR) and either strong co-inhibitory molecule signaling or limited presence of concomitant co-stimulation through CD28. T cell exhaustion occurs after repeated activation of T cells during chronic infection or tumor progression. In acutely cleared infections, a part of activated T cells develops into highly functional memory T cells, while in chronic infections and the tumor microenvironment, the persistent activation of T cells can lead to a gradual development into an exhausted phenotype. This phenotype is defined by poor effector function and sustained expression of inhibitory receptors [14]. While both T cell anergy and T cell exhaustion in natural occurrence are considered reversible, T cell senescence until recently was considered irreversible [15,16,17,18]. Recent studies challenge this distinction by showing that senescent T cells are in fact able to regain function by inhibiting the p38 mitogen-activated protein kinase (MAPK) pathway and show relationships between T cell exhaustion and senescence [19, 20].
Mechanisms of T cell senescence
T cell senescence can be triggered by two major cellular mechanisms: replicative and premature senescence. Replicative senescence is the natural age-related process that occurs after several rounds of proliferation leading to a shortening of telomeric ends. The cell is then put into a senescent state to prevent a potential degeneracy into a cancerous cell. The second mechanism is premature senescence which is a telomere-independent senescence induced by outside factors such as cellular stress [21,22,23]. For example, effector T cells, CD4+ helper, and CD8+ cytotoxic T cells can be forced by Treg cells into senescence, by inducing DNA damage using metabolic competition during cross-talk [22].
Biomarkers for T cell senescence and T cell exhaustion
Although in recent years molecular and cellular biomarkers of effector T cell differentiation have been studied extensively, many of the molecular and signaling pathways related to maturation and senescence of effector T cells are still unknown. T cells in replicative senescence tend to lose co-stimulatory molecules such as CD27 and CD28 while expressing killer cell lectin-like receptor subfamily G (KLRG-1) and CD57. Interfering with the ligation of KLRG-1 on T cells has shown enhanced proliferation capability. CD57 was shown to be associated with severe proliferation impairment and thus is considered the most reliable surface marker for T cell senescence. Furthermore, G1-regulating proteins such as p15, p16, and p21 which are involved in cell cycle regulation and are associated with cellular stress response are upregulated in senescent T cells, with evidence of increased levels of bound p16/Cdk6 and p21/WAF, downregulation of Cdk2 and cyclinD3 expression, and decreased Cdk2 and Cdk6 kinase activity. These molecules inhibit the transition from G1 to S phase forcing cells into a replicative senescence [24,25,26,27,28,29]. Additionally, CD27 and CD28 downregulation is associated with loss of human telomerase RNA component (hTERC) expression, leading to a decrease in telomerase activity and subsequent impaired buildup of telomeric ends [29, 30]. In a recent study, the T cell immunoreceptor with Ig and tyrosine-based inhibitory motif (ITIM) domains (TIGIT) was suggested to be a novel T cell senescence marker. TIGIT was shown to be upregulated in CD8+ T cells of elderly in comparison to young individuals. Moreover, TIGIT+CD8+ T cells exhibited a senescence immunophenotype including high expression of KLRG1 and CD57 while retaining cytotoxicity and function, thus linking the mechanisms of T cell senescence to previous findings pertaining to the role of TIGIT in the mechanism of T cell exhaustion [31, 32]. Furthermore, the negative checkpoint receptor TIGIT was described as a novel marker in exhausted CD4+ and CD8+ T cells after HIV infection [33, 34]. Exhausted T cells hierarchically lose the production of IL-2, their high proliferative capacity and ability for ex vivo killing, followed by loss of production of tumor necrosis factor (TNF), and in the last stage, partial or complete loss of the ability to produce large amounts of interferon-γ which ultimately leads to physical deletion. This decline of effector function is accompanied by a progressive loss of CD4+ T cell help and increased expression of inhibitory receptors, e.g., PD1, CTLA4, TIGIT, LAG-3, CD244, CD160, or TIM3 [35, 36].
T cell senescence progression in aging healthy individuals
Many countries face demographic changes in their population with an over-proportional increase in the elderly in comparison to the young. T cell senescence impairs life-long immune protection and effective vaccination by limiting variability. T cell composition is shifted from undifferentiated naïve T cells to determined memory T cells and further to senescent T cells [4, 13]. The output of naïve T cells decreases after puberty and thymic involution, leading the remaining naïve T cells to progressively become determined and differentiated during lifetime. While the proportion of naïve T cells decreases in early life, the proportion of differentiated memory T cells increase until it reaches a stable plateau during adulthood. After the age of 65, a shift to senescence and an accumulation of highly differentiated CD28− T cells are observed [37]. This accumulation occurs especially strong with respect to the CD8+CD28− T cell subset which expresses enhanced cytotoxicity and regulatory functions while having a shorter replicative lifespan and defective antigen-induced proliferation [28, 37]. There is a growing body of evidence that age-related T cell senescence is not only caused by thymic involution but is also accelerated by memory inflation caused by HCMV infection. HCMV infection is significantly associated with changes in both naïve CD4+ T cell composition as well as memory T cells of the CD8+ subset. Memory inflation leads to an accumulation of HCMV-specific CD8+CD28− T cells which also express typical senescence marker such as KRLG1 and CD57 while remaining highly cytotoxic. This excess expansion of a single HCMV-specific repertoire can occupy up to 50% of the entire CD8+ T cell and 30% of CD4+ T cell compartment of the peripheral blood in HCMV-infected elderly individuals [38,39,40]. This might indicate a joint responsibility of age-related and HCMV-related T cell senescence in the impaired immune response to vaccination as well as an increased susceptibility towards disease and hematological malignancies in elderly individuals. Factors which contribute to T cell senescence and altered T cell subset distribution from young to elderly individuals are shown in Fig. 1.
Schematic model of age and HCMV infection related T cell immunosenescence in the peripheral blood of human. Memory inflation due to latent HCMV infections and thymic involution lead to a shift in T cell distribution from mainly naïve and memory T cells towards effector and senescent T cells with progressing age. Senescent T cells are characterized by shortening of telomeric ends, decrease in telomerase activity, and loss of CD27 and CD28 expression. Markers of T cell senescence include KRLG1, CD57, and the recently identified receptor TIGIT
T cell senescence in hematological malignancies
Malignant tumors utilize many different strategies to evade anti-tumor immunity of the adaptive immune system by creating immunosuppressive microenvironments [39, 40]. Mechanisms of immune evasion include alteration of G1-regulating protein expression, production of suppressive factors like interleukin-10 (IL-10), transforming growth factor beta (TGF-β), and indoleamine-pyrrole 2,3-dioxygenase (IDO) as well as expression of immune inhibitory receptors, e.g., programmed cell death ligand 1 (PD-L1) and recruitment of Treg cells [41,42,43]. An accumulation of senescent CD8+CD28− T cells was observed in several solid tumors, indicating the use of the suppressive activity of senescent T cells as a strategy for immune evasion [44,45,46,47]. Tumor-derived cAMP was shown to be responsible for the direct induction of senescence in T cells and is also a key component of the Treg cell mechanism of forcing T cells into senescence [48]. These findings correlate with re-occurring observations of Treg cell accumulations in hematological malignancies such as acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL), multiple myeloma (MM), and B cell lymphomas [49,50,51,52]. Conclusively, reduced Treg cell accumulation significantly prognosticated low relapse risk and leukemia-free survival (LFS) in AML patients [48, 49]. Recently, senescent T cells including clonally expanded CD8+T cells with a CD28−KLRG1+CD57+ or CD28−CD57+PD-1+phenotype were characterized in MM patients. Remarkably, these T cell clones showed telomere-independent senescence with upregulated telomerase activity indicating reversibility of senescence [50, 51]. Moreover, higher numbers of CD28−CD57+PD-1+T cells were associated with early relapse in patients with MM after autologous stem cell transplantation (ASCT) [50]. In addition, senescent and exhausted T cells in patients negatively affect T cell immunotherapy.
Senescence and exhaustion of CAR-T cells
Currently, ACT is emerging as a potentially curative therapy for patients with advanced hematological malignancies. CAR-T and TCR-T cell therapy makes use of functionally active T cells isolated from patients. These T cells are reconstructed and expanded ex vivo to recognize specific antigens on target cells and are now widely trialed to treat leukemia, lymphoma, and several solid tumors [52,53,54,55,56,57,58]. However, there are functional challenges of engineered T cell therapy in regard to T cell senescence and exhaustion. Firstly, the exposure of T cells from patients to the tumor microenvironment, thus acquiring a senescent and exhausted phenotype, can lead to a progression towards terminal differentiation [59, 60]. PD-1 upregulation within the tumor microenvironment was shown to significantly inhibit T cell function indicating that CAR-T cells, which are produced from T cells with impaired function, might show less effectiveness in targeting leukemia and tumor cells [61,62,63]. Additionally, the endogenous TCR of T cells can have a negative influence on the persistence of CAR-T cells. Presence of TCR antigen when CAR is introduced into T cells with distinct TCR specificity was shown to provoke a loss in CD8+ CAR T cell efficacy associated with T cell exhaustion and apoptosis [64]. Lastly, as demonstrated by Long et al., some signaling from CAR can increase differentiation and exhaustion of T cells, in that tonic CAR CD3ζ phosphorylation, triggered by antigen-independent clustering of CAR single-chain variable fragments, will force early exhaustion of CAR-T cells [65, 66]. Overall, revision of the tumor-related T cell immune senescence and exhaustion are key points in enhancing anti-tumor function in genetically modified T cells.
Strategies to reverse T cell senescence and restore T cell homeostasis in response to aging
There are three main strategies to rejuvenate T cell pools including replacement, reprogramming, and restoration of senescent cells. (1) Replacement strategies include the physical removal of senescent cells from the circulation with the aim of homeostatic expansion of memory and effector T cells. A possible approach is to target and promote selective apoptosis in senescent T cells. In a recent study, an engineered peptide was used to interfere with FOXO4/p53 causing targeted apoptosis in senescent fibroblasts [67], whether this also can be used in inducing apoptosis of senescent T cell remains unknown. Nevertheless, homeostatic expansion in form of autologous stem cell transplantation (ASCT) was shown to successfully reconstitute functional naïve, memory, and effector T cell pools in autoimmune diseases and hematological malignancies [68,69,70,71]. In addition, isolation and banking of cord blood HSCs has been used to reconstitute the immune system for treatment of hematological disorders and may provide hope for homeostatic expansion of functional T cells [72,73,74]. (2) Reprogramming is a promising method to differentiate T cells away from exhausted and senescent states by redifferentiation from T-induced pluripotent stem cells (T-IPSCs) into naive and cytotoxic T cells or dedifferentiation within their own lineage [75,76,77]. Although generation of T cells from human embryonic stem cells (hESCs) and iPSCs was shown to be possible, the TCR repertoire due to seemingly random VDJ gene rearrangements remains unpredictable. Nevertheless, human iPSC-derived T cells transduced with engineered TCRs and CARs specific for tumor antigens were able to infiltrate and delay tumor progression in xenograft models of solid tumors [78]. Moreover, reprogramming can potentially be used for reversion of replicative T cell senescence by enhancing telomerase activity and telomere-length restoration to extend cellular lifespan and prevent telomere-dependent T cell senescence [79, 80]. (3) Restoration strategies aim to restore and maintain the thymic environment thus reversing effects of thymic involution with help of bioengineered thymus organoids in combination with growth-promoting factors and cytokines such as IL-21, which recently was identified as a thymostimulatory cytokine and showed significant immunorestorative function and rejuvenation of the peripheral T cell pool by triggering de novo thymopoiesis in aged mice [81, 82]. Similarly, intrathymic injection of allogenic hematopoietic cells restored functional T cell development after the thymic reconstitution in a mouse model of severe combined immunodeficiency [83]. Preclinical studies have shown generation of thymic organoids from decellularized matrices as an effective approach to rejuvenate the function of T cells and the adaptive immune system. Yet, donor-specific immune tolerance, reproduction of the complex thymic extracellular matrix (ECM), and support of thymic epithelial cells, as well as T cell maturation, remain major challenges [84, 85]. Possible strategies for reversion of T cell senescence and exhaustion to restore T cell homeostasis in response to aging are summarized in Fig. 2.
Replacement, reprogramming, and restoration strategies for reconstitution of senescent and exhausted cells in T cell pool. (1) Replacement: targeting of directed apoptosis in senescent cells as shown by Baar et al. and rebuilding the T cell pool by ASCT. Top left: blockage of P53/FOXO4 binding in fibroblasts using a bioengineered FOXO4-DRI peptide. Top right: collection, purification, and expansion of hematopoietic stem cells, followed by cryopreservation and reinfusion into patient after chemotherapy. (2) Reprogramming: isolation of senescent and exhausted antigen-specific T cells followed by reprogramming into IPSCs, expansion, induction of T cell lineage, and transduction of engineered CAR and TCR before injection. (3) Restoration: preparation of a functional thymus organoid by thymectomy of cadaveric donor, isolation of TEM, transduction with recipient MHC, and recellularization of bioengineered organoid scaffold before transplantation into recipient
Targets for recovery of T cell activation in hematological malignancies
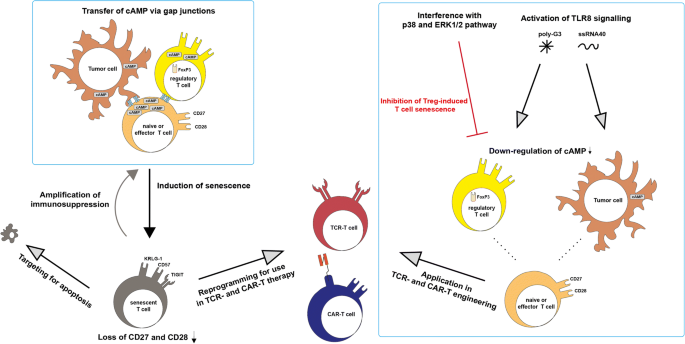
Tumor sites in hematological malignancies were shown to recruit Treg cells and use direct and indirect induction of senescence in their tumor microenvironments as a mechanism of immune suppression [86,87,88,89, 48, 49]. Therefore, one possible target of immunotherapy is the inhibition of tumor-related T cell senescence as well as the possible restoration of senescent T cell function. In cases of increased numbers of CD28−CD57+PD-1+T cells in MM patients, PD-1 blockade was shown to restore proliferation and cytokine secretion in exhausted/senescent CD8+ T cells [87]. Elevated levels of transcription factor B lymphocyte-induced maturation protein 1 (Blimp-1) in patients suffering from AML correlated with upregulation of multiple inhibitory receptors including PD-1 and TIGIT on exhausted, functionally impaired T cells. More importantly, siRNA knockdown of Blimp-1 has shown to reverse the functional defect [90]. cAMP, which is also a key component of Treg cell suppression in aging, is accumulated in tumor sites creating hypoxic microenvironments. Treg and tumor cells in these microenvironments directly induced human naïve T cells and tumor-specific effector T cells to become senescent by increasing cAMP levels using transfer via gap junctions [91, 92]. Due to their inherent suppressive function, dysfunctional senescent T cells then can indirectly maintain the tumor microenvironment and amplify immunosuppression [93], thus indicating that regulation of cAMP level might be a potential approach to revise T cell senescence and disrupt the tumor microenvironment in patients. Recent studies implicate metabolic regulation of tumor cells by Toll-like receptor 8 (TLR8) signaling. Specifically, TLR8 ligands, such as third-generation polyamidoamine dendrimers (poly-G3) and ssRNA40, were shown to enhance antitumor immunity by modulation of endogenous cAMP in tumor cells through the activation of the protein kinase A (PKA) type I–COOH-terminal Src kinase (Csk)–LCK inhibitory pathway [94]. Moreover, ERK1/2 and P38 signaling was identified as regulators of Treg-induced senescent T cells [19, 25]. These results open a possibility to reverse the suppression by tumor microenvironments, creating effector microenvironments by modulation of specific factors in tumor-related T cell senescence. Interestingly, a common alteration in childhood T cell acute lymphoblastic leukemia (T-ALL) cells is the deletion of p16 and p15 and in some cases hypermethylation of a 5′ CpG island in the p15 gene. The accumulation of both proteins is strongly associated with T cell aging and senescence, and thus, their deletion might indicate a role in immortalization and the mechanism of senescence avoidance by some leukemic T cells [24]. Although this indicates that interference with the accumulation of p16 can possibly slow down aging or prevent senescence of T cells, it also potentially harbors an increased risk of provoking T cells to become cancerous and hence should be explored further. Mechanisms of T cell senescence induction in the tumor microenvironment and strategies for revision of T cell senescence for TCR- and CAR-T cell therapy are shown in Fig. 3.
Mechanism of T cell senescence induction in the tumor microenvironment and strategies for revision of T cell senescence for TCR- and CAR-T cell therapy. Treg and tumor cells use cAMP as a key component in senescence induction of naive and effector T cells. Induction of senescence in T cells leads to a loss of CD27 and CD28 and an amplification of immunosuppression in the tumor microenvironment. Interference with p38 and ERK1/2 as well as activation of TLR8 signaling by poly-G3 and ssRNA40 downregulates cAMP levels in Treg and tumor cells, disrupting induction of senescence and immunosuppression. Functional T cells then can be further used in adoptive immunotherapies. Alternatively, senescent T cells can be reprogrammed for dedifferentiation to use in TCR- and CAR-T cell therapies or targeted for apoptosis to help to recover the homeostasis of T cell subsets in patients with hematological malignancies
To improve persistence and effectiveness of CAR-T cells, it is necessary to establish assays to characterize the T cell status in patients who are selected for CAR-T cell therapy. Next, depending on the immune alterations in these patients, different targeting approaches can be chosen to revise senescence and exhaustion as depicted in Figs. 2 and 3. Finally, therapies including PD-1 checkpoint blockade, which can overcome the immune evasion of tumor cells from CAR-T cells within the tumor microenvironment, and the use of apoptosis inhibitor blockade agents, to increase the effect of CAR-T cell therapy, can significantly improve CAR-T cell effectiveness [58, 61, 95, 96].
Conclusion
T cell senescence is playing a key role in immune suppression and evasion of both hematological and solid tumors. Understanding the underlying mechanisms of Treg cell recruitment as well as direct and indirect induction of T cell senescence by tumor microenvironments will open new immunotherapeutic strategies for restoration and recovery of TCR-T and CAR-T cell activation after senescence and exhaustion. Specifically, replacement, reprogramming, and restoration of the immune system as well as modulation of signaling in tumor sites, shifting immunosuppressive microenvironments to become effector microenvironments, are promising approaches. Further, occurrence of potentially reversible telomere-length independent senescent T cells in hematological malignancies has to be investigated more extensively. Understanding of its occurrence might potentially give insight into reversion of replicative T cell senescence for optimized CAR-T or TCR-T cell immunotherapy.
Abbreviations
- ACT:
-
Adoptive cellular immunotherapy
- ALL:
-
Acute lymphoblastic leukemia
- AML:
-
Acute myeloid leukemia
- ASCT:
-
Autologous stem cell transplantation
- Blimp-1:
-
B lymphocyte-induced maturation protein 1
- cAMP:
-
Cyclin adenosine monophosphate
- CAR-T:
-
Chimeric antigen receptor expressing T cell
- CD:
-
Cluster of differentiation
- CLL:
-
Chronic lymphocytic leukemia
- CTLA4:
-
Cytotoxic T-lymphocyte-associated protein 4
- FOXO4:
-
Forkhead box protein O4
- HCMV:
-
Human cytomegalovirus
- hTERC:
-
Human telomerase RNA component
- IDO:
-
Indoleamine-pyrrole 2,3-dioxygenase
- IL-10:
-
Interleukin-10
- ITIM:
-
Immunoreceptor tyrosine-based inhibitory motif
- KLRG-1:
-
Killer cell lectin-like receptor sub family G
- LAG3:
-
Lymphocyte-activation protein 3
- LFS:
-
Leukemia-free survival
- MAPK:
-
Mitogen-activated protein kinase
- MM:
-
Multiple myeloma
- PD-L1:
-
Programmed cell death ligand 1
- PKA:
-
Protein kinase A
- poly-G3:
-
Third-generation poly amidoamine dendrimers
- TCR:
-
T cell receptor
- TGF-β:
-
Transforming growth factor beta
- TIGIT:
-
T cell immunoreceptor with Ig and tyrosine-based inhibitory motif (ITIM) domains
- TIM3:
-
T cell immunoglobulin domain and mucin domain-containing protein3
- T-IPSCs:
-
T-induced pluripotent stem cells
- TLR8:
-
Toll-like receptor 8
- Treg:
-
Regulatory T
References
Dhodapkar MV, Dhodapkar KM. Immune modulation in hematologic malignancies. Semin Oncol. 2015;42:617–25.
Finn OJ. Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol. 2012;23(Suppl 8):viii6–9.
Candeias SM, Gaipl US. The immune system in cancer prevention, development and therapy. Anti Cancer Agents Med Chem. 2016;16:101–7.
van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439–46.
Vicente R, Mausset-Bonnefont A-L, Jorgensen C, Louis-Plence P, Brondello J-M. Cellular senescence impact on immune cell fate and function. Aging Cell. 2016;15:400–6.
Solana R, Tarazona R, Aiello AE, Akbar AN, Appay V, Beswick M, et al. CMV and immunosenescence: from basics to clinics. Immun Ageing. 2012;9:23.
Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–33.
Lindqvist CA, Christiansson LH, Thörn I, Mangsbo S, Paul-Wetterberg G, Sundström C, et al. Both CD4+ FoxP3+ and CD4+ FoxP3– T cells from patients with B-cell malignancy express cytolytic markers and kill autologous leukaemic B cells in vitro. Immunology. 2011;133:296–306.
Lindqvist CA, ASI L. T regulatory cells in B-cell malignancy—tumour support or kiss of death? Immunology. 2012;135:255–60.
Schosserer M, Grillari J, Breitenbach M. The dual role of cellular senescence in developing tumors and their response to cancer therapy. Front Oncol. 2017;7:278.
Singhal SK, Roder JC, Duwe AK. Suppressor cells in immunosenescence. Fed Proc. 1978;37:1245–52.
Leech SH. Cellular immunosenescence. Gerontology. 1980;26:330–45.
Chou JP, Effros RB. T cell replicative senescence in human aging. Curr Pharm Des. 2013;19:1680–98.
Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–9.
Vallejo AN, Weyand CM, Goronzy JJ. T-cell senescence: a culprit of immune abnormalities in chronic inflammation and persistent infection. Trends Mol Med. 2004;10:119–24.
Chappert P, Schwartz RH. Induction of T cell anergy: integration of environmental cues and infectious tolerance. Curr Opin Immunol. 2010;22:552–9.
Baitsch L, Fuertes-Marraco SA, Legat A, Meyer C, Speiser DE. The three main stumbling blocks for anticancer T cells. Trends Immunol. 2012;33:364–72.
Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol. 2013;25:214–21.
Lanna A, Henson SM, Escors D, Akbar AN. The kinase p38 activated by the metabolic regulator AMPK and scaffold TAB1 drives the senescence of human T cells. Nat Immunol. 2014;15:965–72.
Lanna A, Henson SM, Akbar A. The regulation of T cell senescence and metabolism by P38 mapkinase signaling. Innov Aging Oxford University Press. 2017;1:1254.
Campisi J. The biology of replicative senescence. Eur J Cancer. 1997;33:703–9.
Cristofalo VJ, Lorenzini A, Allen RG, Torres C, Tresini M. Replicative senescence: a critical review. Mech Ageing Dev. 2004;125:827–48.
Dock JN, Effros RB. Role of CD8 T cell replicative senescence in human aging and in HIV-mediated immunosenescence. Aging Dis. 2014;2:382–97.
Erickson S, Sangfelt O, Heyman M, Castro J, Einhorn S, Grandér D. Involvement of the Ink4 proteins p16 and p15 in T-lymphocyte senescence. Oncogene. 1998;17:595–602.
Liu X, Mo W, Ye J, Li L, Zhang Y, Hsueh EC, et al. Regulatory T cells trigger effector T cell DNA damage and senescence caused by metabolic competition. Nat Commun. 2018;9:249.
Liu Y, Sanoff HK, Cho H, Burd CE, Torrice C, Ibrahim JG, et al. Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell. 2009;8:439–48.
Qian Y, Chen X. Tumor suppression by p53: making cells senescent. Histol Histopathol. 2010;25:515–26.
Xu W, Larbi A. Markers of T cell senescence in humans. Int J Mol Sci. 2017;18(8). https://doi.org/10.3390/ijms18081742.
Lanna A, Coutavas E, Levati L, Seidel J, Rustin MHA, Henson SM, et al. IFN-α inhibits telomerase in human CD8+ T cells by both hTERT downregulation and induction of p38 MAPK signaling. J Immunol. 2013;191:3744–52.
Bernadotte A, Mikhelson VM, Spivak IM. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging. 2016;8:3–11.
Song Y, Wang B, Song R, Hao Y, Wang D, Li Y, et al. T-cell immunoglobulin and ITIM domain contributes to CD8+T-cell immunosenescence. Aging Cell. 2018;17(2).
Yang Z-Z, Kim HJ, Price-Troska T, Jalali S, Villasboas JC, Novak AJ, et al. Constitutive expression of TIGIT defines a population of CD4+ regulatory T cells in B cell non-Hodgkin lymphoma. J Immunol. 2017;198:155.12.
Tauriainen J, Scharf L, Frederiksen J, Naji A, Ljunggren H-G, Sönnerborg A, et al. Perturbed CD8+ T cell TIGIT/CD226/PVR axis despite early initiation of antiretroviral treatment in HIV infected individuals. Sci Rep. 2017;7:40354.
Chew GM, Fujita T, Webb GM, Burwitz BJ, Wu HL, Reed JS, et al. TIGIT marks exhausted T cells, correlates with disease progression, and serves as a target for immune restoration in HIV and SIV infection. PLoS Pathog. 2016;12:e1005349.
Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–99.
Amezquita RA, Kaech SM. Immunology: the chronicles of T-cell exhaustion. Nature. 2017;543:190–1.
Mou D, Espinosa J, Lo DJ, Kirk AD. CD28 negative T cells: is their loss our gain? Am J Transplant. 2014;14:2460–6.
Tu W, Rao S. Mechanisms underlying T cell immunosenescence: aging and cytomegalovirus infection. Front Microbiol. 2016;7:2111.
Sansoni P, Vescovini R, Fagnoni FF, Akbar A, Arens R, Chiu Y-L, et al. New advances in CMV and immunosenescence. Exp Gerontol. 2014;55:54–62.
Kim J, Kim A-R, Shin E-C. Cytomegalovirus infection and memory T cell inflation. Immune Netw 2015;15: 186–190.
Mougiakakos D, Choudhury A, Lladser A, Kiessling R, Johansson CC. Regulatory T cells in cancer. Adv Cancer Res. 2010;107:57–117.
Chaudhry A, Rudensky AY. Control of inflammation by integration of environmental cues by regulatory T cells. J Clin Invest. 2013;123:939–44.
Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–12.
Syed Khaja AS, Toor SM, El Salhat H, Faour I, Ul Haq N, Ali BR, et al. Preferential accumulation of regulatory T cells with highly immunosuppressive characteristics in breast tumor microenvironment. Oncotarget. 2017;8:33159–71.
Zhang N-N, Chen J-N, Xiao L, Tang F, Zhang Z-G, Zhang Y-W, et al. Accumulation mechanisms of CD4(+) CD25(+) FOXP3(+) regulatory T cells in EBV-associated gastric carcinoma. Sci Rep. 2015;5:18057.
Shang B, Liu Y, Jiang S-J, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep. 2015;5:15179.
Zhang S, Ke X, Zeng S, Wu M, Lou J, Wu L, et al. Analysis of CD8+ Treg cells in patients with ovarian cancer: a possible mechanism for immune impairment. Cell Mol Immunol. 2015;12:580–91.
Ustun C, Miller JS, Munn DH, Weisdorf DJ, Blazar BR. Regulatory T cells in acute myelogenous leukemia: is it time for immunomodulation? Blood. 2011;118:5084–95.
Sander FE, Nilsson M, Rydström A, Aurelius J, Riise RE, Movitz C, et al. Role of regulatory T cells in acute myeloid leukemia patients undergoing relapse-preventive immunotherapy. Cancer Immunol Immunother. 2017;66:1473–84.
Chung DJ, Pronschinske KB, Shyer JA, Sharma S, Leung S, Curran SA, et al. T-cell exhaustion in multiple myeloma relapse after autotransplant: optimal timing of immunotherapy. Cancer Immunol Res. 2016;4:61–71.
Suen H, Brown R, Yang S, Weatherburn C, Ho PJ, Woodland N, et al. Multiple myeloma causes clonal T-cell immunosenescence: identification of potential novel targets for promoting tumour immunity and implications for checkpoint blockade. Leukemia. 2016;30:1716–24.
Im A, Pavletic SZ. Immunotherapy in hematologic malignancies: past, present, and future. J Hematol Oncol. 2017;10(1):94.
Yu S, Li A, Liu Q, Li T, Yuan X, Han X, Wu K. Chimeric antigen receptor T cells: a novel therapy for solid tumors. J Hematol Oncol. 2017;10(1):78.
Sun W. Recent advances in cancer immunotherapy. J Hematol Oncol. 2017;10:96.
Lichtenegger FS, Krupka C, Haubner S, Köhnke T, Subklewe M. Recent developments in immunotherapy of acute myeloid leukemia. J Hematol Oncol. 2017;10:142.
Wei G, Ding L, Wang J, Hu Y, Huang H. Advances of CD19-directed chimeric antigen receptor-modified T cells in refractory/relapsed acute lymphoblastic leukemia. Exp Hematol Oncol. 2017;6:10.
Zhang C, Liu J, Zhong JF, Zhang X. Engineering CAR-T cells. Biomark Res. 2017;5:22.
Qin L, Zhao R, Li P. Incorporation of functional elements enhances the antitumor capacity of CAR T cells. Exp Hematol Oncol. 2017;6:28.
Ye B, Stary CM, Li X, Gao Q, Kang C, Xiong X. Engineering chimeric antigen receptor-T cells for cancer treatment. Mol Cancer. 2018;17(1):32.
Yao D, Xu L, Tan J, Zhang Y, Lu S, Li D, et al. Re-balance of memory T cell subsets in peripheral blood from patients with CML after TKI treatment. Oncotarget. 2017;8(47):81852–9.
Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016;126(8):3130–44.
Guha P, Cunetta M, Somasundar P, Espat NJ, Junghans RP, Frontline Science KSC. Functionally impaired geriatric CAR-T cells rescued by increased α5β1 integrin expression. J Leukoc Biol. 2017;102(2):201–8.
Fan M, Li M, Gao L, Geng S, Wang J, Wang Y, et al. Chimeric antigen receptors for adoptive T cell therapy in acute myeloid leukemia. J Hematol Oncol. 2017;10:151.
Yang Y, Kohler ME, Chien CD, Sauter CT, Jacoby E, Yan C, et al. TCR engagement negatively affects CD8 but not CD4 CAR T cell expansion and leukemic clearance. Sci Transl Med. 2017;9(417). https://doi.org/10.1126/scitranslmed.aag1209.
Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21(6):581–90.
Gomes-Silva D, Mukherjee M, Srinivasan M, Krenciute G, Dakhova O, Zheng Y, et al. Tonic 4-1BB costimulation in chimeric antigen receptors impedes T cell survival and is vector-dependent. Cell Rep. 2017;21(1):17–26.
Baar MP, Brandt RMC, Putavet DA, Klein JDD, Derks KWJ, Bourgeois BRM, et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell. 2017;169:132–147.e16.
Delemarre EM, van den Broek T, Mijnheer G, Meerding J, Wehrens EJ, Olek S, et al. Autologous stem cell transplantation aids autoimmune patients by functional renewal and TCR diversification of regulatory T cells. Blood. 2016;127:91–101.
Delemarre EM, Roord STA, van den Broek T, Zonneveld-Huijssoon E, de Jager W, Rozemuller H, et al. Brief report: Autologous stem cell transplantation restores immune tolerance in experimental arthritis by renewal and modulation of the Teff cell compartment. Arthritis Rheum. 2014;66:350–6.
Rueff J, Medinger M, Heim D, Passweg J, Stern M. Lymphocyte subset recovery and outcome after autologous hematopoietic stem cell transplantation for plasma cell myeloma. Biol Blood Marrow Transplant. 2014;20:896–9.
Farge D, Arruda LCM, Brigant F, Clave E, Douay C, Marjanovic Z, et al. Long-term immune reconstitution and T cell repertoire analysis after autologous hematopoietic stem cell transplantation in systemic sclerosis patients. J Hematol Oncol. 2017;10:21.
Rizk M, Aziz J, Shorr R, Allan DS. Cell-based therapy using umbilical cord blood for novel indications in regenerative therapy and immune modulation: an updated systematic scoping review of the literature. Biol Blood Marrow Transplant. 2017;23:1607–13.
Damien P, Allan DS. Regenerative therapy and immune modulation using umbilical cord blood–derived cells. Biol Blood Marrow Transplant bbmtorg. 2015;21(9):1545–54.
Politikos I, Boussiotis VA. The role of the thymus in T-cell immune reconstitution after umbilical cord blood transplantation. Blood. 2014;124:3201–11.
Karagiannis P, Iriguchi S, Kaneko S. Reprogramming away from the exhausted T cell state. Semin Immunol. 2016;28:35–44.
Nishimura T, Kaneko S, Kawana-Tachikawa A, Tajima Y, Goto H, Zhu D, et al. Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. Cell Stem Cell. 2013;12:114–26.
Crompton JG, Clever D, Vizcardo R, Rao M, Restifo NP. Reprogramming antitumor immunity. Trends Immunol. 2014;35:178–85.
Themeli M, Kloss CC, Ciriello G, Fedorov VD, Perna F, Gonen M, et al. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat Biotechnol. 2013;31:928–33.
Effros RB. Telomerase induction in T cells: a cure for aging and disease? Exp Gerontol. 2007;42:416–20.
Allsopp R. Telomere length and iPSC re-programming: survival of the longest. Cell Res. 2012;22:614–5.
Al-Chami E, Tormo A, Pasquin S, Kanjarawi R, Ziouani S, Rafei M. Interleukin-21 administration to aged mice rejuvenates their peripheral T-cell pool by triggering de novo thymopoiesis. Aging Cell. 2016;15:349–60.
Tormo A, Khodayarian F, Cui Y, Al-Chami E, Kanjarawi R, Noé B, et al. Interleukin-21 promotes thymopoiesis recovery following hematopoietic stem cell transplantation. J Hematol Oncol. 2017;10:120.
Tuckett AZ, Thornton RH, O’Reilly RJ, den Brink MRM v, Zakrzewski JL. Intrathymic injection of hematopoietic progenitor cells establishes functional T cell development in a mouse model of severe combined immunodeficiency. J Hematol Oncol. 2017;10:109.
Tajima A, Pradhan I, Trucco M, Fan Y. Restoration of thymus function with bioengineered thymus organoids. Curr Stem Cell Rep. 2016;2:128–39.
Fan Y, Tajima A, Goh SK, Geng X, Gualtierotti G, Grupillo M, et al. Bioengineering thymus organoids to restore thymic function and induce donor-specific immune tolerance to allografts. Mol Ther. 2015;23:1262–77.
Wu C-P, Qing X, Wu C-Y, Zhu H, Zhou H-Y. Immunophenotype and increased presence of CD4(+) CD25(+) regulatory T cells in patients with acute lymphoblastic leukemia. Oncol Lett. 2012;3:421–4.
Idris S-Z, Hassan N, Lee L-J, Md Noor S, Osman R, Abdul-Jalil M, et al. Increased regulatory T cells in acute lymphoblastic leukemia patients. Hematology. 2015;20:523–9.
Zelle-Rieser C, Thangavadivel S, Biedermann R, Brunner A, Stoitzner P, Willenbacher E, et al. T cells in multiple myeloma display features of exhaustion and senescence at the tumor site. J Hematol Oncol. 2016;9:116.
Serag El-Dien MM, Abdou AG, Asaad NY, Abd El-Wahed MM, Kora MAE-HM. Intratumoral FOXP3+ regulatory T cells in diffuse large B-cell lymphoma. Appl Immunohistochem Mol Morphol. 2017;25:534–42.
Zhu L, Kong Y, Zhang J, Claxton DF, Ehmann WC, Rybka WB, et al. Blimp-1 impairs T cell function via upregulation of TIGIT and PD-1 in patients with acute myeloid leukemia. J Hematol Oncol. 2017;10:124.
Lang PO, Govind S, Aspinall R. Reversing T cell immunosenescence: why, who, and how. Age. 2013;35:609–20.
Ye J, Huang X, Hsueh EC, Zhang Q, Ma C, Zhang Y, et al. Human regulatory T cells induce T-lymphocyte senescence. Blood. 2012;120:2021–31.
Ye J, Peng G. Controlling T cell senescence in the tumor microenvironment for tumor immunotherapy. Oncoimmunology. 2015;4:e994398.
Ye J, Ma C, Hsueh EC, Dou J, Mo W, Liu S, et al. TLR8 signaling enhances tumor immunity by preventing tumor-induced T-cell senescence. EMBO Mol Med. 2014;6:1294–311.
Huang X, Yang Y. Driving an improved CAR for cancer immunotherapy. J Clin Invest. 2016;126(8):2795–8.
Karlsson H. Approaches to augment CAR T-cell therapy by targeting the apoptotic machinery. Biochem Soc Trans. 2016;44:371–6.
Acknowledgements
We thank Anusha Aravamudhan for additional proofreading of the manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (nos. 91642111, 81770152) and the Guangdong Provincial Basic Research Program (nos. 2015B020227003, 201807010004, 201803040017).
Availability of data and materials
The material supporting the conclusions of this review is included within the article.
Author information
Authors and Affiliations
Contributions
YQL designed the review. DK drafted the manuscript and prepared the figures. LX and YQL helped to modify the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kasakovski, D., Xu, L. & Li, Y. T cell senescence and CAR-T cell exhaustion in hematological malignancies. J Hematol Oncol 11, 91 (2018). https://doi.org/10.1186/s13045-018-0629-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13045-018-0629-x